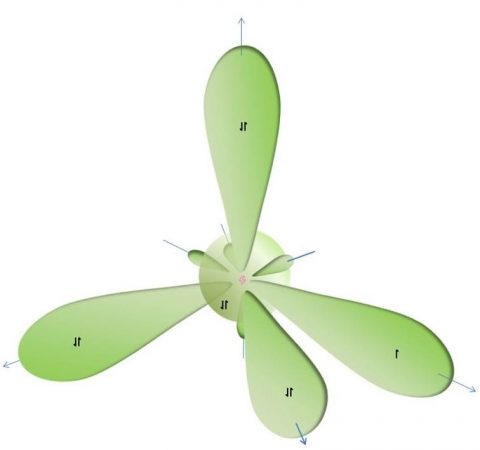
Fluorine atom showing its 1S and 2S/2P electron orbitals (hybridized) with atomic nucleus at centre
[Editor’s note: With no further ado, and with no introduction necessary, here is a second post from Craig Pearcey; Witness his science and despair, quacks of the world!]
First for the basic chemistry
There is one particular word that tends to get many CAM supporters very vocal and the conspiracists thinking about running for their home-made bunkers in a basement somewhere. It is the word “fluorine” or any of its analogues. However, before getting into their anti-fluorine claims, we need to briefly review it properties as both an element and in various molecular forms. Without this background it may be possible to make certain assumptions about fluorine that are baseless for a given molecular structure and application. It is this very type of error that the antivaxxers and many in the CAM field make in regards thimerosal – i.e. Ethyl(2-mercaptobenzoato-(2-)-O,S) mercurate(1-) sodium, methyl-mercury, and elemental mercury. All three have significantly differing chemical properties based on their molecular structure, but some individuals/groups continue to attribute the toxicity and chemical properties for elemental mercury to thimerosal (Ethyl(2-mercaptobenzoato-(2-)-O,S) mercurate(1-) sodium). Similarly, drawing conclusions on thimerosal toxicity from methyl mercury is equally flawed. In addition, in their instance in equating the three they refuse to provide any viable mechanism how thimerosal is converted into either elemental mercury or methyl mercury, or how they can be attributed the same chemical properties.
And now for fluorine chemistry
Fluorine is one of the halides (i.e. halogen), and is located in Period 2 (the second row of the periodic table), Group 17 / Group VIIa (the column second most from the right of the table). It has the highest electronegativity of any of the elements (i.e. a “love” for accumulating extra electrons) in the periodic table, and consequently is one of the strongest oxidants in nature. This strong oxidizing potential tends to make it dangerous to any living organism that comes into contact with it in it elemental form (typically as molecular fluorine – F2) due to its tendency to cause severe oxidative damage. Fluoride on the other hand, the anion of fluorine, is supplied in various forms for the prevention of cavities. These forms include sodium fluoride, fluorosilicic acid, and sodium fluorosilicate. As an anion, i.e. F–, its potential for oxidation is spent and it tends to form salts like sodium fluoride (NaF) with any cation in its immediate environmental. However, sodium fluoride is still toxic in small to moderate amounts (the lethal dose for a 70kg/154lb individual is 5-10 grams) and has a health warning of three on the NFPA 704 scale. In addition, sodium fluoride is also highly soluble in water. Lithium fluoride on the other hand, is far less water soluble and is typically associated with molten salts, and is used for (among other things) solar energy conduction in solar plants. Fluorosilicic acid (H2SiF6) and sodium fluorosilicate (Na2SiF6) are the acid and conjugate base of the same fluoridated silicon chemical species. Both are highly toxic (given by the NFPA 704 scale numbers of 3 and 2 respectively) and fluorosilicic acid is corrosive, including corrosive to glass. When elemental fluorine is chemically bonded to organics, its high electronegativity allows it to form strongly bonded associations with the organic backbone. This allows multi-fluoro-alkyls to have considerable thermodynamic stability, which lends them to multiple commercial and industrial uses (eg. high performance fire-retardants), particularly when multiple or complete hydrogen-fluorine substitution has been effected such as the various perfluor-alkyls and polyfluoror–alkyls (PFAS).
Turning Flint waters into a merry-go-round…
Given the prior discussion on heavy metals, it is appropriate at this point to move onto another issue Natural News writers often frequent – even though it does not include the topic of fluoride. Most recently, the writers of Natural News have published the an article entitled:- “City governments across America are poisoning children with lead and nothing is being done about it” in which they reference what is now known as the Flint Water Crisis. It is one of a number of articles in the similar vane in which they attempt to link the Flint Water crisis and the hazardously high lead levels in in Flint’s (Michigan) public water system to their unfounded claim of deliberate poisoning of the general US public with elevated levels of heavy metals in all US public and water drinking water. The whole story of the Flint Water crisis is known by most of the US public (though little known outside of the US) and has been extensively covered in the US press. In essence, the metropolitan of Flint changed over its water supply from the Detroit Water and Sewerage Department to the Flint River, but did not add the necessary corrosion inhibiters to the Flint river water supply. This resulting in hazardously elevated levels of lead in the public water supplied to Flint due corrosion / leaching of lead out from the old lead-pipe based public water system into the public water. The effects on the citizens of Flint, particularly the poorer, were severe with as many as 6000-12000 children experiencing health related problems. In addition to, that when the problem became openly known, there were attempts by certain individuals to hide evidence of negligence. At present, extensive efforts have been undertaken to address the problem, include the changing back to the Detroit Water and Sewerage Department water supply, replacing the old lead pipe-lines with something more modern and infrastructure upgrades. Legal action is also being presumed against certain individuals. In addition, the story of the Flint Water Crisis is still developing as these efforts are ongoing. In general, Natural News’ coverage thereof is within acceptable bounds until they move beyond the basic facts of the case. Once, the writers of Natural News attempt to link the events of the Flint Water Crisis to their broader conspiratorial claims of heavy-metal poisoning of the general US public, they move from a factual position to one of unsupported supposition. The general mountainous preponderance of Water Quality testing data/evidence in the US, by the EPA and others, does not support they preposition. In fact the avalanche of evidence actually supports the counter claim. This cannot be more clearly demonstrated than by the fact that Mr Adams’ own CWC laboratory results (a review of which was undertaken earlier) strongly suggests no heavy metal poisoning of the general US public , but overall; adequate to good quality drinking water instead.
RED-ALERT! PFAS invasion on the way …
With this background, it is appropriate at this juncture to mention three recent articles on the Natural News website as they illustrate the approach in regards the general topic of anything to do with fluorine by many CAM supporters and conspiratists. The first of these was written by Mr. Mike Adams (Health Ranger) and is entitled “Millions of Americans exposed to deadly, toxic chemicals in public water supplies… massive scientific investigation backs up Health Ranger findings“. In his article, Mr. Adams states:
The study comes on the heels of the Natural News nationwide water quality assessment effort which analyzed hundreds of water samples via ICP-MS instrumentation to determine that 6.7% of the U.S. water supply is contaminated with toxic heavy metals that exceed allowable EPA limits. Click here to read the results of my own study in the Natural Science Journal, the journal I launched to publish real science in the public interest (without corporate or government influence). My article explaining the illegal heavy metals contamination found in the U.S. water supply is detailed at this link.
This article was subsequently followed reasonably quickly by two further articles on Natural News regarding the same topic and are entitled “Millions of Americans at risk for cancer and other harmful diseases due to toxic tap water” and “California tap water most toxic in nation; Harvard study finds deadly industrial chemicals used to fight fires, insulate pipes and more“. All three articles refer to the same recent joint study undertaken by the Harvard T.H. Chan School of Public Health and the Harvard John A. Paulson School of Engineering and Applied Sciences in which the lead author, Xindi Hu, discussed the progressive emergent threat of PFAS in US public drinking water as part of her doctoral thesis. The author of the Harvard study used data supplied by the EPA’s Third Unregulated Contaminant Monitoring Rule (UCMR3) program and a brief review of part of the contents thereof indicates that the Harvard study itself was well designed and executed. However, it is clear from both the title and the quoted contents of Adams’ article that he is trying to ram through his claims of heavy metals poisoning of the US public via its drinking water on the coattails of Hu’s completely unrelated study. A prior debunking of Mr. Mike Adams’ prior claims around heavy-metal poisoning of US public and state water is available on Science-Based Medicine. The impression that all three articles attempt to produce is that the EPA is either neglectfully ignorant of the risks associated with PFAS contamination of the national water supply, or criminally inactive about it. However, neither impression is true as, firstly, the EPA has been aware of the risks associated with PFAS for some time as given by the EPA’s UCMR3 testing program used in the Harvard study and, secondly, they have instituted activities to deal with the threat. In other words, the EPA has not been caught suddenly by surprise, and they are already at work tracking and attempting to address the problem. Furthermore, the issue of PFAS is not just a US problem, as the three Natural News articles appear to imply, but a global problem. This is clearly evident in the considerable amount of work and investigation being undertaken by the UNEF under the UN Global Monitoring Plan on POPs. Data from these and many other sources show similar levels of PFAS’s in the ground water, fauna and populous of all EU countries as compared to the USA. A similar trend is also observed around the rest of the globe. Lastly, given Mr. Adams related claim, a final comparison of the maps provide for heavy-metal results and the EPA on PFAS clearly suggests that there is no significant correlation between Adams’ heavy-metal data and the EPA PFAS data. In the end, these three Natural News articles are nothing more than an attempt to appeal to authority to justify their unfounded claims of US public water heavy-metal poisoning using a good-quality study that is not at all related to their claims – a “guilty by association” approach.
Beware the dental hygiene!
In addition to these three articles on the Natural News website on PFAS, a pair of articles on fluoride and dental hygiene were also been published during the same period. As with the three on PFAS, the central theme has been the issue of the apparent toxicity of US state and national drinking water, but this time in regards the addition of various sources of fluoride with a view to improving dental hygiene. These articles include “Whiten your teeth naturally, without chemicals” and “How New Zealand is systematically poisoning its citizens with fluoride… while crushing local control over water safety” and if you are really curious about what Mr Mike Adams’ is actually think on the subject, you can find a whole category of articles on fluoride. Overall, the general tone given by Natural News is that the writers believe that the use of fluoride at any dosage level (even the smallest) is extremely toxic and harmful to any individual regardless of the method of delivery or the frequency of the dose. There are a few references on Natural News to dental fluorosis and skeletal fluorosis as well as frequent references to avoiding fluoride or filtering it out as given by the titles: “Water purification breakthrough revealed: AquaTru countertop water purifier removes 128 toxic chemicals, fluoride, heavy metals and more” and “Big Berkey beats ProPur gravity water filter for removal of heavy metals and toxic elements“. It is almost as though they are talking about high intensity radioactive contaminants like Cobalt-60 or Iodine-131. Most recently, the writers of Natural News have also published the an article entitled:- “How to prevent fluoride from destroying your brain” which refers to a study undertaken by Dr. Philippe Grandjean (Harvard School of Public Health) and Dr. Philip Landrigan (Icahn School of Medicine published) in the medical journal The Lancet (Neurology)- see abstract and full article. In their article, Natural News asserts:-
In 2014, the journal Lancet Neurology declared that fluoride is a developmental neurotoxin. A meta-analysis of 27 cross-sectional studies was lead by Dr. Philippe Grandjean of the Harvard School of Public Health, and Dr. Philip Landrigan of the Icahn School of Medicine. The study analyzed the effects fluoridation had on children across the world, though most were from China. What the researchers found was shocking. Exposure to elevated levels of fluoridation led to a decrease in IQ of about seven points, on average. Most of the water supplies in the studies had fluoride levels that would be permissible by current EPA standards, of less than 4mg per liter.
However, these views by the writers of Natural News are unsupported scientifically and have no basis in reality. The science behind the application of fluoride to teeth for good dental health has been extensively studied and is well established. Among the aspects covered by these studies is the chemical mechanics behind the conversion of hydroxyapatite to fluoro-hydroxyapatite in tooth enamel with fluoride application, as well as chemical/solubility equilibrium studies (Le Châtelier’s principle) and reaction kinetic studies associated hydroxyapatite deposition/re-deposition – including some more recent studies. The impact of dosage levels has also been well studied and from these studies, the appropriate and safe levels for fluoride application have been determined (0.7 – 1.2 ppm in drinking water). These studies have also shown that dental fluorosis and skeletal fluorosis only occur at high and extremely high levels respectively, typically at consumption levels one or more orders of magnitude above those proposed (i.e. 10 or even 100 ppm), at which point community water supplies would begin removing fluoride from the water. Thus, the claim by the Natural News writers that fluoride application at any dosage is dangerous is without any basis. In regards the Lancet study, the writers of Natural News appear to have quoted the Lancet study completely out of context. The language and structure of Natural News article suggests that its writers are attempting to create the impression that the Lancet article was sole about fluoride and its dangers, specifically in regards that added to US drinking water. However, the original Lancet article is actually a study that focuses on a large variety of known neurotoxicants (not just fluoride) and how they impact on cognitive development, with a specific emphasis on children. Some of the neurotoxins referenced in the article are Lead , polychlorinated biphenyls (PCB) , arsenic, toluene, manganese, chlorpyrifos, dichlorodiphenyltrichloroethane, tetrachloroethylene, and polybrominated diphenyl ethers. Fluoride is only mention twice in the article, once in table 2 and once in the abstract, however, it is not discussed at all in the article, including in the abstract. Thus, it is difficult to know the context of the fluoride (ie. the dosage level) that the article is referring to. A study of the sources sighted by Dr Gandjean and Dr Landrigan in the original article is more revealing. One of the sources referenced is an article published in the journal of Environmental Health Perspectives that deals with developmental delay in respect to fluoride dosage levels that are abnormally high as given by an article entitled:- “Developmental Fluoride Neurotoxicity: A Systematic Review and Meta-Analysis” . An extract from the articles abstract states:-
Conclusions: The results support the possibility of an adverse effect of high fluoride exposure on children’s neurodevelopment. Future research should include detailed individual-level information on prenatal exposure, neurobehavioral performance, and covariates for adjustment.
Lastly, the mention of 4.0 ppm (4.0mg/L) as the current EPA standard by the Natural News writers appears to be a break-down in understanding and general confusion around the NCR 2006 study (which was into the adverse impact of fluoride at the 2-4 ppm level) and the stipulated EPA specifications for fluoride in drinking water (0.7 – 1.2 ppm).
Further afield …
Two other claims that have been made by those in the anti-fluoridation camp are:
- That the rest of the world is against the use of fluoride to dental hygiene entirely, and
- That fluoride aids the uptake/release of heavy metals in the human body – thus aiding heavy metal poisoning.
In regards the rest of the world being against fluoridation, this is completely untrue as both the UN World Health Organization and EU European Food Safety Authority are strongly in favour thereof. This, then, raises the question as to why considerably more countries do not add fluoride to their drinking water than do. The answer is that the majority of the countries whom do not add fluoride to their public water use other methods to provide fluoride to their general public, such as fluoride in toothpaste, oral medications, and dental fluoride treatments typically involving pastes/gels. The reasons behind this are based more political and legal issues, not scientific or health ones. In regards higher heavy-metal uptakes/release by fluoride, the proponents hereof have not provided any viable scientific evidence for these claims, nor have they ever proposed a viable chemical mechanism for this. The single article they reference mentions only increased lead levels in the water supply to certain areas of Flint due to the increased corrosion of lead pipes via fluorosilicate.
Final words on PFAS and fluoride
So, in summary:
- PFAS are a clear point of concern in the US, the EU and globally. Presently, the EPA and UN are carefully monitoring PFAS levels and working to address these concerns. However, for the Natural News writer to try link their unfounded claims of heavy-metal poisoning of the general US public via their drinking water with the PFAS study is completely without merit and inherently false.
- The use of fluoride for dental health is well documented and thoroughly scientifically proven, provided the dosage of fluoride is maintain within the appropriate range. For the writers of Natural News to claim the use of fluoride at any dosage level, by any mechanism, and at any frequency, to be dangerous and damaging to public health, is totally unfounded and pure misinformation.
- Although most of the world does not use fluoridation of water as a means of supplying fluoride to the general populous, they generally are in favour of fluoride application for oral hygiene and supply it in other forms.
- The CAM and conspiracy camps have not provided a viable chemical mechanism to explain how fluoride worsens the uptake/release of heavy-metals by the human body to facilitate poisoning thereby.
An unrelated bit of fun chemistry
And now for something completely off topic and of interest in regards Period 2 chemistry. Being in Period 2, each fluorine atom processes only s and p-orbitals (i.e. electron shells), but no d or f-orbitals which gives fluorine some unique properties. Consequently, this is why (together with the number of protons in its nucleus against its size) fluorine has the highest electronegativity of all known elements. This lack of d and f-orbitals also tend to confer unique properties to all the other Period 2 elements such as boron, carbon, nitrogen and oxygen, ect. This unique s/p-only orbital property for carbon as well as its four-valence bonding capacity makes it the only element in the universe that can readily and easily form polymers and chains of endless length that are thermodynamically stable. Silicon, the next four-valence bonding element in the Periodic Table (Period 3), does not readily form long polymers due to it having d-orbitals which favour silicon-oxygen linkages. Consequently, silicon tends to form silanes and silicones polymers, but only to a given size due to steric hindrance of the polymer structure. It is for this reason that any possible extra-terrestrial life (if it exists) on any exoplanets (include those recently discovery by either radial-velocity or transit methods – see the Kepler satellite) will always be carbon based rather than silicon based (despite science fiction’s general enthusiasm for silicon based life-forms).
